Certain food emulsifiers trigger chronic intestinal inflammation, which also occurs in various metabolic diseases. They work by creating imbalances in the gut microbiota, which promote the proliferation of mobile and inflammatory bacteria. A team Insert from the Cochin Institute in Paris succeeded in protecting mice from these harmful mechanisms using an original approach: immunization against a protein that is necessary for the mobility of the contaminated microbiota bacteria.
Food emulsifiers are additives used by the food industry to improve the texture of certain processed products. Among them we find carboxymethylcellulose (identified by the code name E466), which acts as a thickener and stabilizer, or polysorbate 80 (P80), which prevents the formation of deposits by facilitating the mixing of the ingredients. These substances can be found in dairy products, soups, sauces, dessert creams, etc. These molecules are very useful for manufacturers, but they still pose a public health problem: various studies, including several by Benoît Chassaing, Inserm researcher at the Cochin Institute in Paris, showed that the consumption of these two emulsifiers increases the composition of the intestinal microbiota disturbs and promotes chronic inflammation of the intestine, a phenomenon that is associated with metabolic disorders. “ These substances alter the balance between populations of microorganisms naturally occurring in the gut microbiota, leading to an excess of pro-inflammatory bacteria », explains the researcher.
Mobile bacteria
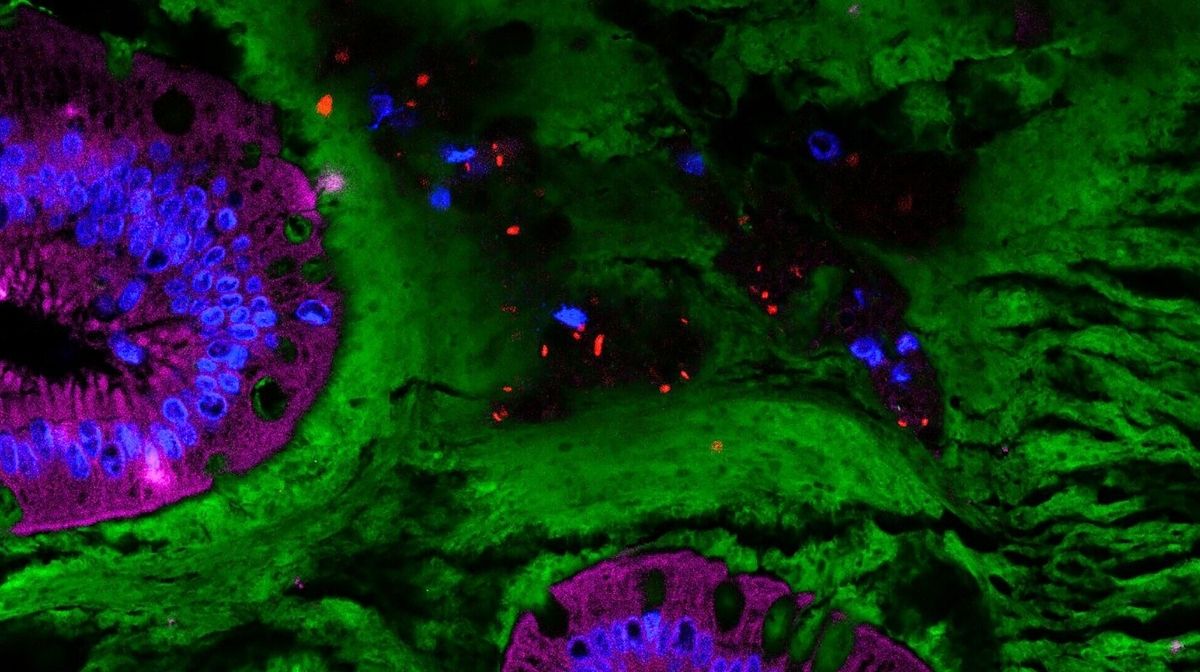
The latter have the peculiarity of being equipped with flagella, small dynamic filaments that make them mobile and able to penetrate the mucous layer that covers the intestinal wall. However, its function is precisely to protect the rest of the body from substances present in the digestive tract by preventing it from entering the bloodstream. “ The mucus invasion by certain bacteria from the intestinal microbiota leads to inflammation not only locally, but also systemically, i.e. spread to the rest of the body. All of these mechanisms contribute to the development of various diseases such as obesity, type 2 diabetes or inflammatory bowel disease (IBD). With our studies based on the use of emulsifiers, we aim to advance the understanding of the interactions between the microbiota and its host and the disorders that lead to these pathological situations.he explains. The aim is to successfully contain this invasive phenomenon and prevent its consequences. »
An anti-flagellin vaccine
To achieve this, Benoît Chassaing and his team hypothesized that by blocking the flagella of the bacteria that possess them, they would lose their ability to penetrate the intestinal mucus and thus become less inflammatory to the body. To test this hypothesis, the researchers vaccinated rodents against flagellin, the protein that makes up flagella. Specifically, the animals received repeated doses of this purified protein injected directly into their stomachs. “ These injections cause the production of anti-flagellin antibodies, which block the synthesis of bacterial flagella. », clarifies Benoît Chassaing. The rodents then consumed emulsifiers and the researchers then analyzed their microbiota. If imbalances between the different microorganism populations could still be observed in the vaccinated animals, the amount of bacteria equipped with flagella in the intestinal mucus was significantly reduced or even reduced to zero. As a result, the extent of local inflammation in these mice was lower than in non-immunized animals that had also received the emulsifiers.
“ This vaccination approach therefore protects well against bacterial invasion of the intestinal mucus and associated inflammation. Of course, our idea is not to vaccinate individuals against the effects of food additives, but the possibility of administering recombinant flagellin seems interesting for combating chronic intestinal inflammation.explains the researcher. Our work aims to advance preventive approaches against inflammatory mechanisms caused by changes in the gut microbiota, regardless of the cause, to reduce the risk of various metabolic diseases. »
Benoît Chassaing is responsible for the team Interactions between microbiota and mucus in chronic inflammatory diseases at the Cochin Institute in Paris (Unit 1016 Inserm/CNRS/Université Paris Cité).
source : M. Kordahi et al. Microbiota motility vaccination protects mice from the deleterious effects of consuming dietary emulsifiers. PLoS Biology from September 19, 2023; doi: 10.1371/journal.pbio.3002289
author :AR
also read