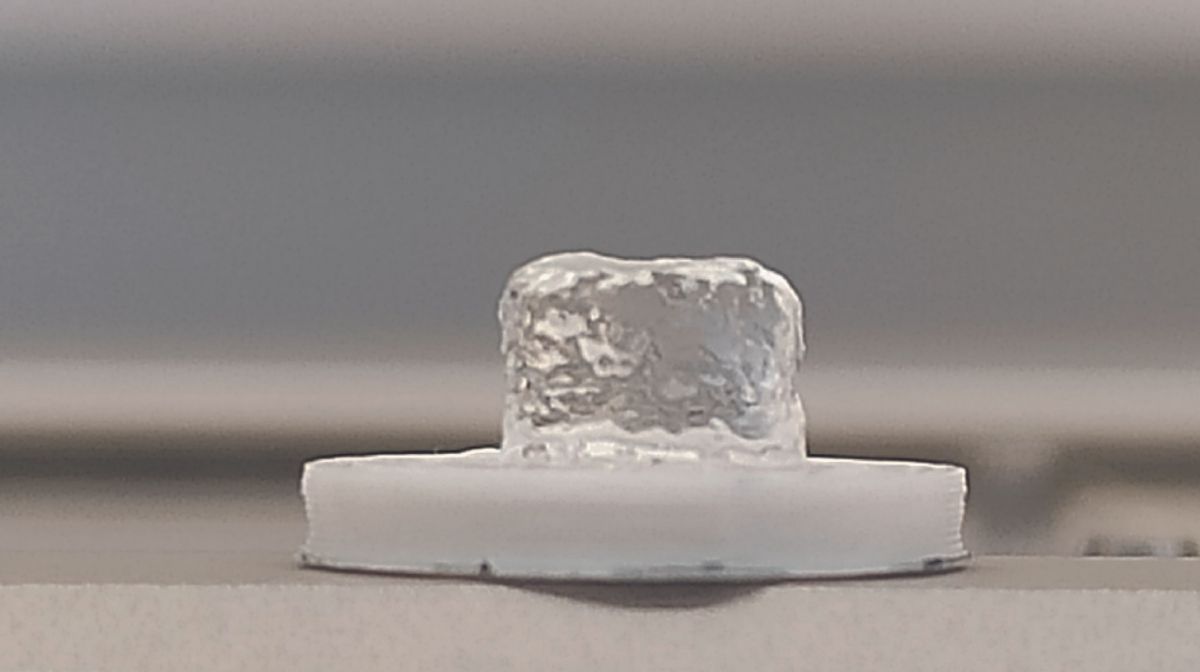
The promise of bioprinting is to assemble living tissue in three dimensions. Beyond clinical use, this manufacturing process also enables the development of scalable experimental models to better understand how our bodies function and the occurrence of diseases or to evaluate the effect of new therapeutic strategies.
An article that can be found in the magazineInsert #59

Bioprinting promises to revolutionize the medicine of tomorrow. Based on 3D printing, these additive manufacturing processes enable the layer-by-layer construction of biological tissue in the laboratory. These can be used in the context of so-called “regenerative” medicine, but also to develop innovative experimental models. Many types of models are now essential for research to develop and validate hypotheses, but many are based on complex animal experiments. In order to limit their use, several cell culture techniques have been developed since the mid-1920se Century. They have enabled great advances in medicine and biology, but encounter several pitfalls, most notably the fact that the three-dimensional environment of the cells is not reproduced in these 2D cultures. In addition, cells interact not only with each other, but also with each other extracellular matrix, this network of long molecular chains that organizes tissue. To overcome these limitations and best mimic the conditions under which cells develop in tissues, the use of 3D bioprinting is a very attractive strategy. “ These object shaping technologies enable the organization of cells and a synthetic extracellular matrix in space to reproduce the biological functions of natural tissues. », explains Vianney Delplace, chemist and Inserm researcher in Nantes.
Hydrogels to mimic biological tissues
There remains a need for printable materials that are compatible with the presence of living cells and whose composition and physical properties are close to those of tissues. To meet this technological challenge, one category of materials is generating great interest in the biomedical world: hydrogels. “ Consisting of a network of chains Polymers When hydrated, these biomaterials have high water concentration and viscoelastic properties that mimic the natural environment of cells », explains Vianney Delplace. Together with his colleagues, the researcher managed to produce a hydrogel based on hyaluronic acid polymer naturally present in the extracellular matrix, which retains its shape after flowing. This allowed them to print objects of a specific shape in the presence of cells. Second, the research group managed to adjust the properties of these objects after printing. “ We added small molecules to the hydrogel that react with other complementary molecules previously immobilized on the hyaluronic acid polymers. Their reactions make it possible to change the composition of the hydrogel, increase its rigidity or improve the adhesion of cells to the synthetic matrix. » These modifications could be made at different times and therefore make it possible to change the parameters of these biomaterials, so-called “dynamic clickable bioinks”, in the fourth dimension. “ This is a first step in that direction In vitro modeling Diseases or degeneration and aging processes », estimates Vianney Delplace.
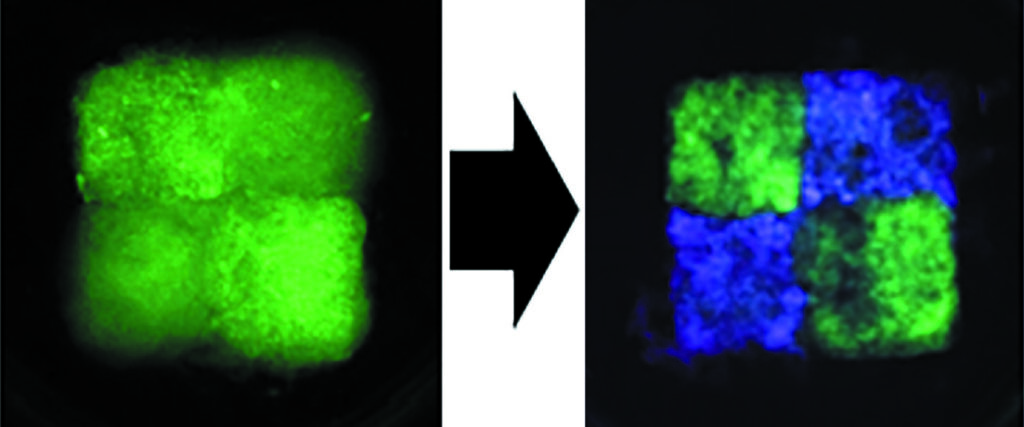
Modeling the development of osteoarthritis
The young researcher has also just received funding from the National Research Agency to develop such an experimental osteoarthritis model. This joint disease, which is characterized by a significant breakdown of cartilage and causes pain, stiffness and loss of mobility, cannot currently be treated. Modeling the osteoarthritis joint in the laboratory would therefore make it possible to better understand the degenerative processes occurring in the joint and to test therapy strategies developed in the laboratory. For this project, called DYNAM – OA, Vianney Delplace and his colleagues will attempt to print three different types of fabric in the same experimental model. As a matter of fact, “ In osteoarthritis, all tissues of the joint are affected, not only the cartilage, but also the membrane synovialwhich produces a fluid that allows good lubrication of the joint and the subchondral bone, which is located between the cartilage and the bone of the jointexplains the researcher. Once this model is validated, we will induce a degenerative process under culture conditions to mimic osteoarthritis. in vitro to study how different tissues communicate and interact over time. »
Welcome to the fourth dimension
In addition to osteoarthritis, so many pathologies could be modeled through 4D bioprinting. “ Once this technology is developed, it will be possible to create simple, controlled and modifiable models over time that can be followed repeatedly to study the function of normal or pathological tissues, as well as the biological processes associated with them. », confirms Baptiste Charbonnier, materials specialist and Inserm researcher in the same laboratory. The latter is also working on the development of multi-material bone and gum models to better understand periodontal diseases, which are the most common cause of tooth loss worldwide. In addition to a better understanding of biological mechanisms, these models also enable the testing of therapeutic approaches while limiting animal testing. “ There is now great interest in biology and medical research in generating model tissues for ethical questions. », emphasizes Baptiste Charbonnier. The work of the Nantes researchers therefore opens great perspectives for biomedical research, which should quickly use this tool to understand the functioning and malfunctions of our bodies, and in a more ethical way.
Vianney Delplace and Baptiste Charbonnier are all Inserm research officers for the unit Regenerative medicine and the skeleton (RMeS, Unit 1229 Inserm/University of Nantes/Oniris), in Nantes.
source : P. Tournier et al. Clickable dynamic bioinks enable temporally and spatially controlled changes in construct composition and mechanical properties after printing. Adv. Science (wine)., September 15, 2023; doi: 10.1002/advs.202300055
Author: SP
also read